How to Find Number of Moles
The mole symbol mol is the unit of amount of substance in the International System of Units SI. The mole is related to the mass of an element in the following way.

Molarity M The Concentration Of A Solution As The Number Of Moles Of Solute Chemistry Education Biology Notes Chemistry
For example if you know there are 335 x 10 22 water molecules in a gram of water and want to find how many moles of water this is.

. This equates to a 5-ounce mole eating 50 pounds of its prey in a year. Start for free now. Molarity moles of solute liters of solution 12 mol CaCl 2 2905 L 0413080895.
Find out the molar mass of the substance hint. Find the molar mass of the substance. It is derived from the amount of atoms in 12 g of the isotope carbon-12.
Number of moles formula is. The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. One mole of carbon-12 atoms has 602214076 10 23 atoms and a mass of 12 grams.
Molarity is the number of moles of a substance in one litre of solution. Finally click on calculate. Pennsilfaani officially the Commonwealth of Pennsylvania is a state spanning the Mid-Atlantic Northeastern and Appalachian regions of the United States.
Definition of Number of Moles. Divide 2 by 6817 and you have 00293 moles of NH 4 2 S. For one mole the atomic or molecular mass will be the same as the weight.
Calculate how many moles are mentioned in the question. The formula for the number of moles formula is expressed as. Also you can make use of the free grams to moles calculator to calculate the number of moles instantly and precisely.
Does an increased number of moles correlate to a higher risk of melanoma. This number is represented by the subscript next to the element symbol in the molecular formula. Just like the way it is easier to measure intergalactic Space distance in light-years relatively to measure it in inches.
Number of moles 95 8694. There are a number of castor oil products sold that are advertised to repel moles and voles. Moles are expert diggers that will consume up to 60 to 100 of their body weight in insects grubs and earthworms each day.
It refers to a huge number that we use to measure atoms. Originally a mole was the quantity of anything that has the same number of particles found in 12000 grams of carbon-12. If you know the quantity of mole it can be converted into grams and vice.
Determine the number of moles in 95g of MnO 2. DNA calculations to convert µg to pmol for double-stranded and single-stranded DNA convert micrograms of DNA to pmol ends calculate vectorinsert molar ratio and convert OD260 readings to µgml. Depending on what the substance is an elementary entity may be an.
Multiply both the values. Due to the temporary residual nature of. You can use Molar mass of the substance alone to calculate molar mass.
Texas ˈ t ɛ k s ə s also locally ˈ t ɛ k s ɪ z. To go from moles to molecules multiply the number of moles by 602 x 10 23. For example imagine you have 2 g of NH 4 2 S and you want to convert it to moles.
In 1 kg-moles of any substance there exists exactly 103. In comparison one mole of oxygen consists by definition of the same number of atoms as carbon-12 but it has a mass of 15999 grams. Texas Tejas is a state in the South Central region of the United StatesAt 268596 square miles 695662 km 2 and with more than 291 million residents in 2020 it is the second-largest US.
We know the atomic mass of calcium is 40. The molecular mass of NH 4 2 S is 6817gmol. This is defined as 0001 kilogram per mole or 1 gram per mole.
Pennsylvania ˌ p ɛ n s ɪ l ˈ v eɪ n i ə PEN-səl-VAY-nee-ə. M nV where n is the number of moles and V is the volume in litres. Mass of one mole MnO 2 8694g.
Using a calculator divide the number of grams by the molar mass. Divide the number of moles by the number of liters. Award winning educational materials like worksheets games lesson plans and activities designed to help kids succeed.
Now that you have the number of liters you can divide the number of moles of solute by this value in order to find the molarity of the solution. Moreover it is equal to the number of atoms in 12 grams of carbon-12 that is just about 6022 10 23 atoms. To go from molecules to moles divide the numbers of molecules by 602 x 10 23.
The result is the number of moles in your element or compound. Also calculate molarity of solutions perform molar conversions calculate dilutions and perform other calculations common in molecular biology labs. The reason is that one mole of the substance contains the amount of moles that are exactly in 12 grams of the carbon-12.
State by both area after Alaska and population after CaliforniaTexas shares borders with the states of Louisiana to the. Multiply each elements atomic mass by the number of atoms of that element in the molecule. The number of moles of KMnO4 will be 0158.
Is the number of particles usually atoms or molecules of the gas. Find average molecular mass. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams per mole of oxygen 159994 grams per mole and of chlorine 35453 grams per mole.
Its a lot easier to write the word mole. The mole is defined as containing exactly 6022 140 76 10 23 elementary entities. Check out photos of normal molesincluding pink moles red moles flat moles and raised molesand see a wide variation of appearances.
How many atoms in a mole. To work it out find the atomic or molecular mass of your substance and multiply it by the number of moles you have. Since a molecule is just a collection of atoms you can add the masses of the atoms together to find the mass of the molecule.
If you use the average atomic masses instead of the mass of a specific isotope the answer is the average mass of the molecule as found in a naturally occurring sample. They are often larger than other moles and have an abnormal shape or color. One mole consists of Avogadro number of atoms.
The official symbol for molarity is c concentration but many people use the old symbol M. That number of particles is Avogadros Number which is roughly 602x10 23A mole of carbon atoms is 602x10 23 carbon atoms. A mole of chemistry teachers is 602x10 23 chemistry teachers.
N M V Example. The number of atoms or other particles in a mole is the same for all substances. See Signs and Symptoms of Melanoma Skin Cancer for descriptions of how moles and melanomas look They can appear on skin that is exposed to the sun.
The molar mass of KMnO4 is 158032 gmol. Convert 250 grams of KMnO4 to moles. We can rearrange this equation to get the number of moles.
Number of moles Mass of substance Mass of one mole. Mass of MnO 2 95g. Multiply the relative atomic mass by the molar mass constant.
Solved Examples On Number Of Moles Formulas. These moles look a little like normal moles but also have some features of melanoma. It borders Delaware to the southeast Maryland to the south West Virginia to the southwest Ohio to the west Lake Erie and the Canadian.
How many moles are there in 1kg. Add these values together for each different atom in the molecule. Find the number of moles.
There are 60221407610 23 atoms in a mole. What Benign Moles Look Like. Atypical moles dysplastic nevi.
Divide the given mass 250 g by the molar mass 158032 gmol to get the moles. In SI units p is measured in pascals V is measured in cubic metres n is measured in moles and T in kelvins the Kelvin scale is a shifted Celsius scale where 000 K 27315 C the lowest possible temperature. Formula to calculate number of moles weightatomic mass of the element 8040 2 Hence number of moles in calcium is 2 In our below online number of moles calculator enter the weight and select the element from the drop-down menu.
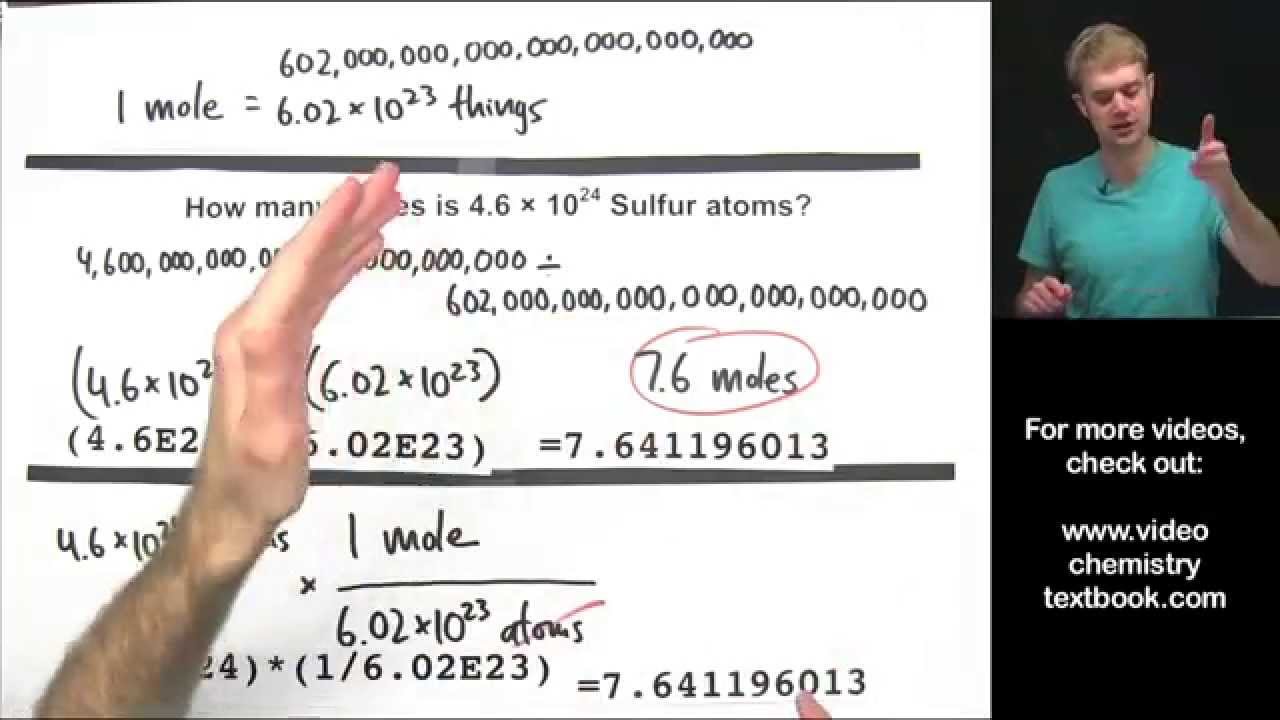
Converting Between Moles Atoms And Molecules Teaching Chemistry Chemistry Textbook High School Chemistry

The Mole Mole Molecules Meant To Be

Partial Pressures Of Gases And Mole Fractions Chemistry Tutorial Mole Fraction Chemistry Fractions


Comments
Post a Comment